top of page
The transducer's main role in a biosensor is to transfer the change caused by the target analyte and bioreceptor's interaction into an electric signal that can be interpreted by scientists. Transducers are mainly in close contact with the bioreceptors and can detect small changes in light, heat pH, mass, and electricity. By converting the change into a signal, the transducer ultimately helps researchers learn how much of what has been detected. Listed below are the categories in which transducers place into and how they 'sense' the change between target analyte and bioreceptor interaction.
ELECTROCHEMICAL TRANSDUCERS
When the target analyte binds to the bioreceptor and triggers a change involving electricity, electrochemical transducers are used to convert the change into an electrical signal. There are several ways that a biosensor can measure electrochemical changes by using one of the four electrochemical transducers listed below:
Amperometric transducers can detect changes by measuring deviations in electrochemical oxidation or reduction of the bioreceptors. This is done by applying a potential on two electrodes; the working electrode which is in direct contact with the bioreceptor and therefore changes, and the reference electrode which remains at a fixed value. Electrodes are electricity conductors, and while the working electrode is made out of gold, carbon, or platinum, the reference electrode is silver. The current created indicates the bioreceptor interaction since it is a direct measure of the rate of electron transfer.
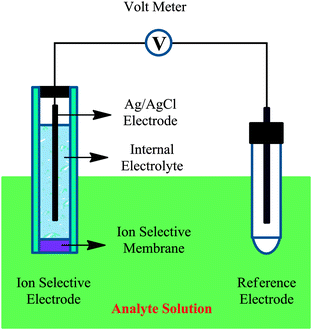
Potentiometric transducers measure the charge aggregation and potential that the target analyte brings when it binds to the bioreceptor. The potential developed is between these two electrodes, as the interactions with the ion selective electrode raise the charged potential compared to the reference electrode which is unaffected by the solution. A high impedance voltmeter is used to calculate this potential and must use a formula known as the Nerst equation to see what the concentration of the analyte is compared to the transducer's signal.
Impedance transducers use electrochemical impedance spectroscopy to measure the resistive changes made by the biorecognition event. Current is applied before and after the interaction using a three-electrode system and the transducer detects how much obstruction the analyte binding has caused to the sinsusoidal electrical wave. This change in impedance directly measures the concentration of the target analyte.
Conductometric transducers detect change by measuring the difference in conductive properties of a sample before and after the biorecognition event has taken place. The interaction changes the ion concentration which leads to a change to the solution electrical conductivity. Two electrodes are once again used, and an ohmeter is employed to measure the change in conductance.
OPTICAL TRANSDUCERS
This transducers employ photons (light particles) to gather information about the target analyte. The transducer uses either the change of optical diffraction or electrochemiluminescence to detect the analyte's presence and concentration. Many techniques can be employed such as fluorescence and fiber optics to obtain the measurements. Evanescent field detection principle, which allows the detection of fluorophores that are close to the optic fiber, is widely used in these transducers.
ION SENSITIVE TRANSDUCERS
Field effect transistors (FETs) can be combined with an ion sensitive surface to measure the change in surface electrical potential, since the biorecognition event triggers slight changes in it. This transducer consists of a electrode with a polymer layer that is permeable to the ions from the analyte. The advantage to using this transducer is that the output signal is directly porportional to the analyte concentration. ISFET transducers are best known to detect pH changes in a sample.
PIEZOELECTRIC TRANSDUCERS
Piezoelectric transducers devise a signal based on the change of mass of its membrane. When the target analyte binds to the bioreceptor, a slight change in mass occurs, and piezoelectric transducers measure that to reveal the molecule's concentration. These transducers normally utilize thin piezoelectric quartz crystals as either "resonating crystals (QCM), or as bulk/surface acoustic wave (SAW) devices," which are two methods of detection through change of mass.
CALORIMETRIC TRANSDUCERS
Pyroelectric transducers measure the heat from the analyte + bioreceptor reaction to give out its porportional signal. When the temperature changes, the transducer generates a current and the total heat measured by these transducers is directly porportional to the molar enthalpy, which is the energy released or absorbed in the form of heat. This then also equals the total number of molecules engaged in the reaction, which is basically the analyte's concentration. The temperature change is most often detected by a enzyme thermistor.







(I-9)
(I-16)
(I-10)
(I-11)
(I-12)
(I-13)
(I-14)
(I-15)
bottom of page